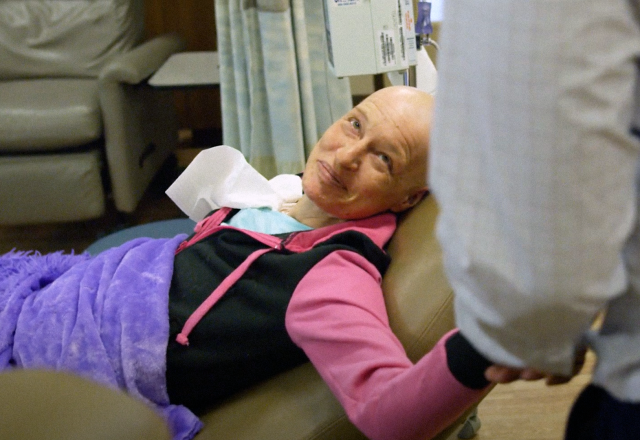
Medical Oncology
Our medical oncologists coordinate and direct your medical care in collaboration with your referring physician and other specialists.
Find out moreSurveillance Clinic
Our acute care clinic serves the urgent needs of all HOAF patients experiencing problems or symptoms related to their cancer treatment or diagnosis.
Find out moreResearch & Clinical Trials
HOAF patients have access to the most advanced cancer care available, including clinical trials.
Find out moreIron Replacement
We offer intravenous iron replacement therapy for our cancer and blood disorder patients.
Find out moreInfusion
Our facilities offer a range of innovative infusion therapies including chemo-biotherapy, immunotherapy, antibiotics, and other specialty drug infusions.
Find out moreHematology
We provide hematology services, including the treatment of coagulation and clotting disorders as well as diseases related to blood and bone marrow.
Find out moreCopay Assistance
We understand the financial burden of cancer care on our patients and their families. HOAF will work with you to coordinate copay assistance whenever possible.
Find out moreChemotherapy
We offer multiple cancer treatment options from traditional chemotherapy to advanced immuno- and biotherapies.
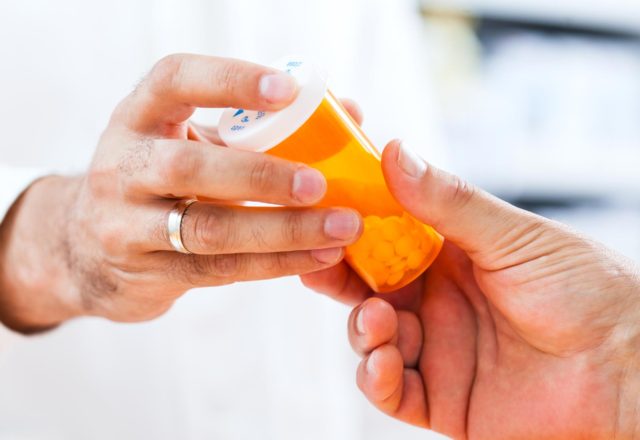
Find out morePrescription Services
For our patients’ convenience, HOAF provides on-site prescription services for oral therapies, as well as any supportive medicines you may need during your care.
Find out more